오므론에 자주 문의 주신 질문사항은 FAQ로 정리하여
빠른 답변을 제공하고 있습니다. FAQ에서 빠르게 답변을 확인해보세요.
Our
Technology
1973년부터 시작된 오므론 혈압계는 끊임없는 기술개발과 혁신적인 제품을 선보이고자 노력하고 있습니다.
혁신을 위한 오므론의 도전은 계속됩니다.
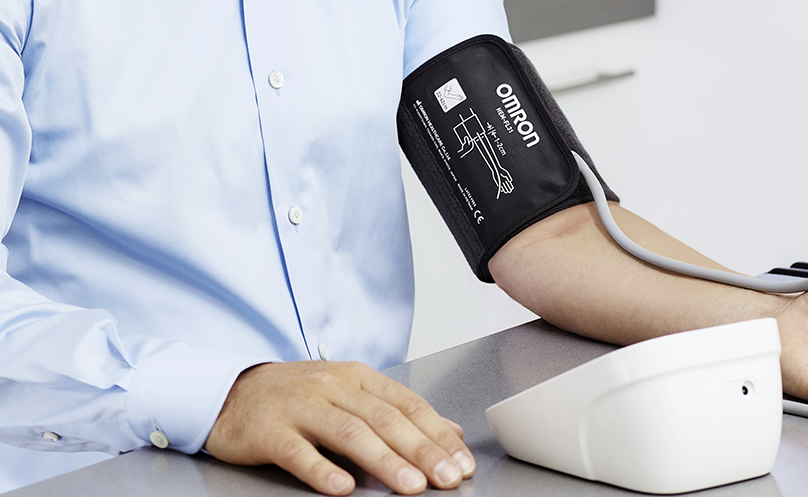
- 국제기준으로 검증한
오므론 자동전자 혈압계 - 오므론 혈압계는 AAMI(미국의료기기협회),
ESH(유럽고혈압학회) 등 국제적 검증 프로토콜을
준수하여 압력 정확도를 검증 받았습니다.

- 혈압 측정의 정확성을
향상 시키는 기술 인텔리센스 - 오므론 혈압계는 특허 받은 혈압 측정 기술인 인텔리센스
(Intellisense) 기술을 사용하여, 측정 시 혈압계가 자동으로
측정자의 호흡과 팔의 둘레, 동맥의 혈류 진동 세기를 감지하여 한
사람 한 사람에 맞춰 커프의 가압량과 감압 속도를 자동 조정하기
때문에 보다 정확하고 편안한 혈압측정이 가능합니다.


주식회사 후지경제 < 글로벌 가전 시장 총 조사 2025 > (2024년 실적)
가전용 전자 혈압계 세계 누계 판매수(2025년 9월 시점)
- 50년 이상 전통의
글로벌 브랜드 - 오므론 혈압계는 1973년 첫 혈압계 런칭 이후, 전 세계 130개국
이상의 국가에서 판매되고 있으며, 혈압계 제조사로서는 최초로
2025년에 4억 대 판매를 돌파함으로써 전세계 환자와 의료
전문가들에게 인정받고 있음이 증명되었습니다. 세분화된
측정자의 니즈를 만족시키기 위해 폭넓은 라인업을
갖추고 있으며, 심혈관질환 관리 분야에서 최고의 기술과
솔루션을 제공하기 위해 혁신을 지속하고 있습니다.
많은 분들이 혈압계를 선택하실 때 가장 중요하게 생각하는 것이 ‘정확도’입니다.
혈압계의 정확도는 국제적인 검증 프로토콜을 준수하였는지의 여부로 확인할 수 있습니다.
프로토콜 검증은 독립적인 기관인 AAMI (Association for the Advancement of Medical Instrumentation) 및
유럽 고혈압 협회 (European Society for Hypertension, ESH) 와 같은 공식적인 기관에서 엄격한 테스트로 평가한 후, 프로토콜 통과 여부가 결정됩니다.
국내에서 판매되는 오므론의 모든 혈압계는 적어도 1개 이상의 국제적 검증 프로토콜을 준수하여 압력 정확도를 검증받았습니다.




